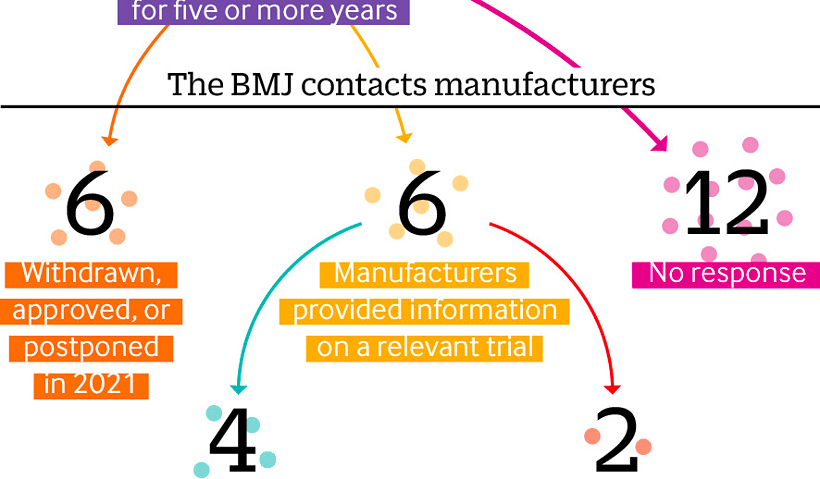
By Elisabeth Mahase Taken Directly from the BMJ doi: https://doi.org/10.1136/bmj.n1898 Criticisms of the US Food and Drug Administration’s accelerated approval process have resurfaced after the recent…
View More FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway