By Elisabeth Mahase
Taken Directly from the BMJ doi: https://doi.org/10.1136/bmj.n1898
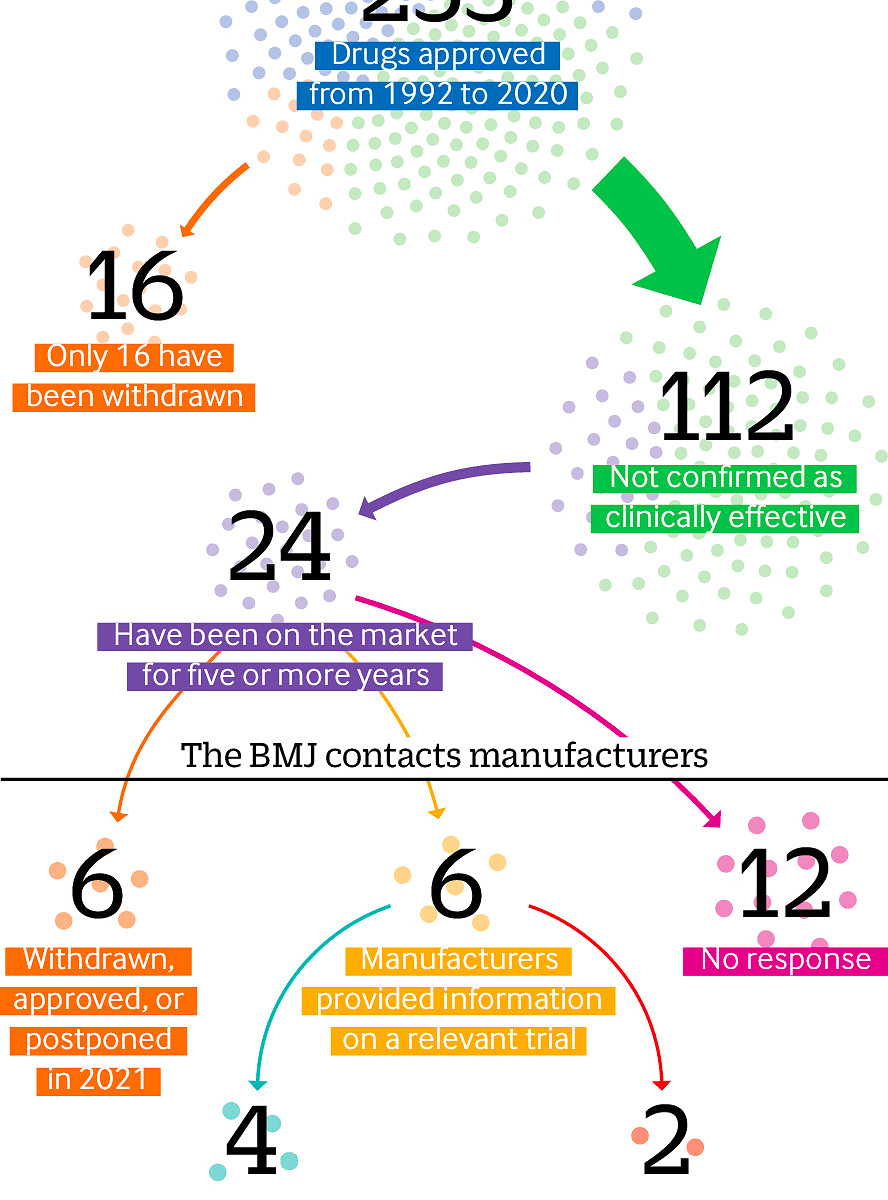
Criticisms of the US Food and Drug Administration’s accelerated approval process have resurfaced after the recent approval of aducanumab (Aduhelm) for dementia. Elisabeth Mahase finds that the process is plagued by missing efficacy data and questionable evidence.
Read the full article here: https://www.bmj.com/content/374/bmj.n1898
Related
FDA calls for investigation into industry influence during Alzheimer’s drug approval Published: 12 July 2021; BMJ 374 doi:10.1136/bmj.n1778
FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness Published: 08 June 2021; BMJ 373 doi:10.1136/bmj.n1462