By Dr Timothy Swinn
Edited by Dr Saadia Aslam
The PROACT-Xa trial (Prospective Randomized On-X Anticoagulation Clinical Trial)1 was stopped early due to higher incidence of valve thrombosis/valve-related thromboembolism with apixaban when compared to warfarin (INR target range 2.0-3.0) for patients with a mechanical On-X aortic valve.
This multi-centre assessor-blind trial aimed to investigate whether apixaban was non-inferior to warfarin for prevention of thrombo-embolic events for patients with an aortic On-X mechanical valve.
The On-X valve is considered low thrombosis risk. The PROACT trial demonstrated that patients with an aortic On-X valve on warfarin with an INR target range 1.5-2.0 (plus low-dose aspirin) did not have a higher incidence of valve thrombosis than those with INR target range 2.0-3.02. Based on this, the 2020 American Heart Association guidelines recommended adopting an INR target range 1.5-2.0 plus aspirin (75-100mg daily) for patients with an On-X AVR and no additional thromboembolic risk factors3.
PROACT-Xa included 863 participants, with an On-X aortic valve implanted at least 3 months prior, between May 2020 and September 2022 (433 randomised to apixaban, 430 to warfarin with target INR 2.0-3.0, 94% were co-prescribed aspirin 81mg once daily). The primary endpoint was a composite of valve thrombosis and valve-related thromboembolic complications. This occurred at a rate of 4.2%/patient-year (95% confidence interval [C.I.] 2.3-6.0) in the apixaban group and 1.3%/patient-year (95% C.I. 0.3-2.3) in the warfarin group and the decision was taken to stop the trial at this point due to the higher rate in the apixaban group. All participants were then switched onto warfarin. Figure 1 (taken from the paper) shows the cumulative incidence of primary endpoint1.
Apixaban had a lower rate of major bleeding in the trial, but this did not reach statistical significance (Hazard ratio 0.6; 95% C.I. 0.3-1.3). Other trials investigating apixaban vs. warfarin with the same INR target for AF have shown reduced major bleeding4.
This trial has demonstrated that apixaban is not non-inferior to warfarin (and likely would have been shown to be inferior had the trial continued), even with a lower-thrombosis risk valve in the aortic site. This may change in the future with mechanical valves that are even less thrombogenic, however further research is required before changes in clinical practice are implemented.
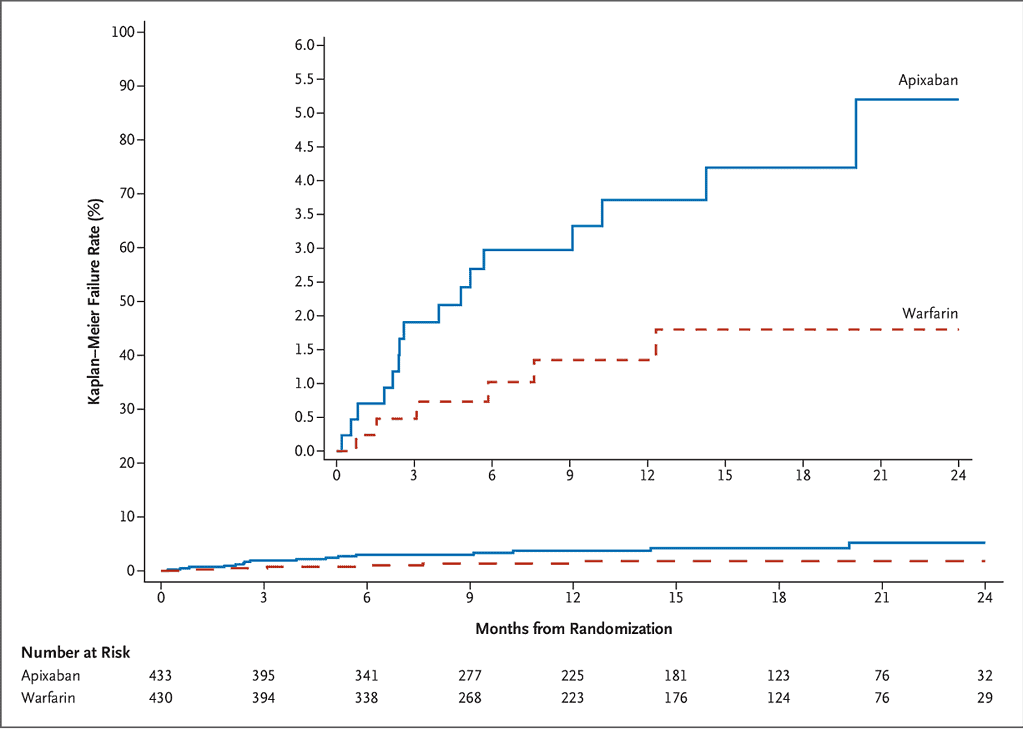
Figure 1: Cumulative incidence of valve thrombosis or valve-related thromboembolism1.
References
1. Wang TY, Svensson LG, Wen J, Vekstein A, Gerdisch M, Rao VU, et al. Apixaban or Warfarin in Patients with an On-X Mechanical Aortic Valve. NEJM Evid. 2023 May 6;0(0):EVIDoa2300067.
2. Puskas JD, Gerdisch M, Nichols D, Fermin L, Rhenman B, Kapoor D, et al. Anticoagulation and Antiplatelet Strategies After On-X Mechanical Aortic Valve Replacement. J Am Coll Cardiol. 2018 Jun 19;71(24):2717–26.
3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021 Feb 2;143(5):e72–227.
4. Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ. 2018 Jul 4;362:k2505.